
Clinical trials for hemophilia
This handbook contains basic information about clinical trials for hemophilia. The WFH does not engage in the practice of medicine
Year: 2022
Language: English
Author(s): World Federation of Hemophilia
For people considering participation in a clinical trial, it is extremely important to learn as much as possible about the clinical trial, the care expected, the cost, and the team that will be conducting the trial. It is expected that you will have questions, and essential that your questions are answered. It may be helpful to write down a list of questions to ask before meeting with a clinical trial research team.
The WFH does not engage in the practice of medicine and under no circumstances recommends particular treatment for specific individuals. For diagnosis or consultation on a specific medical problem, the WFH recommends that you contact your physician or local treatment centre. Before administering any products, the WFH urges patients to check dosages with a physician or hemophilia centre staff, and to consult the pharmaceutical company’s printed instructions.
The WFH does not promote any particular pharmaceutical product and any mention of any commercial brand in this presentation is strictly for educational purposes.

This handbook contains basic information about clinical trials for hemophilia. The WFH does not engage in the practice of medicine
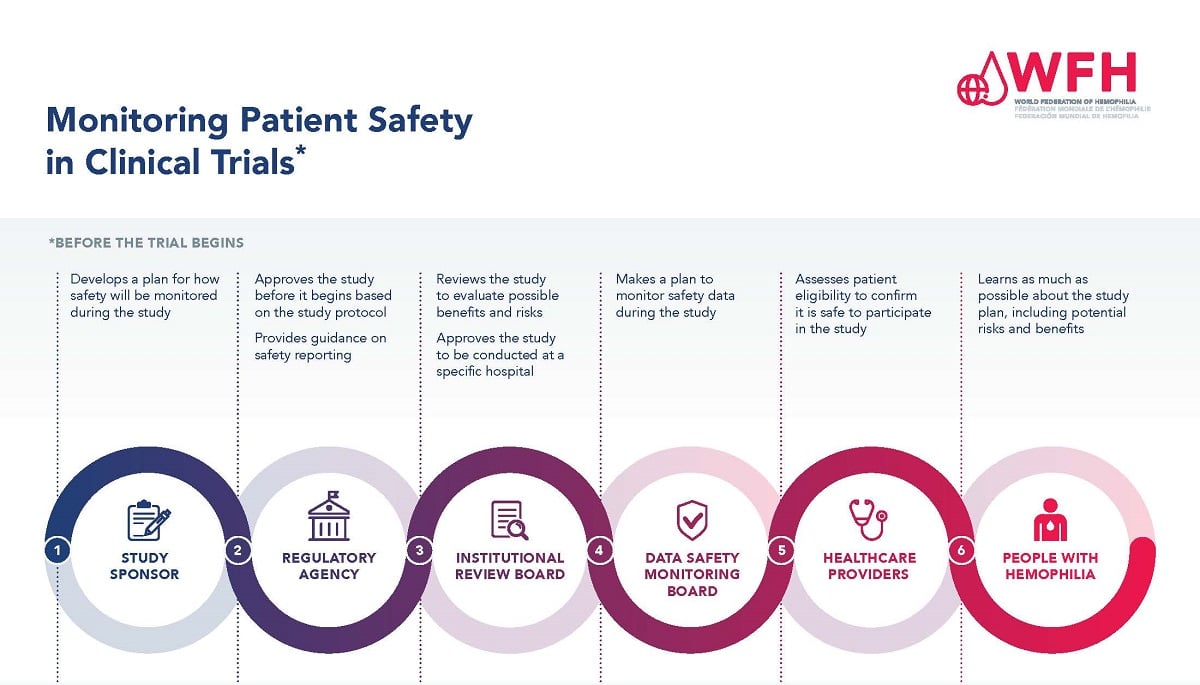
Ensuring patient safety is of paramount importance during the clinical trial process. There are many levels of study approval and
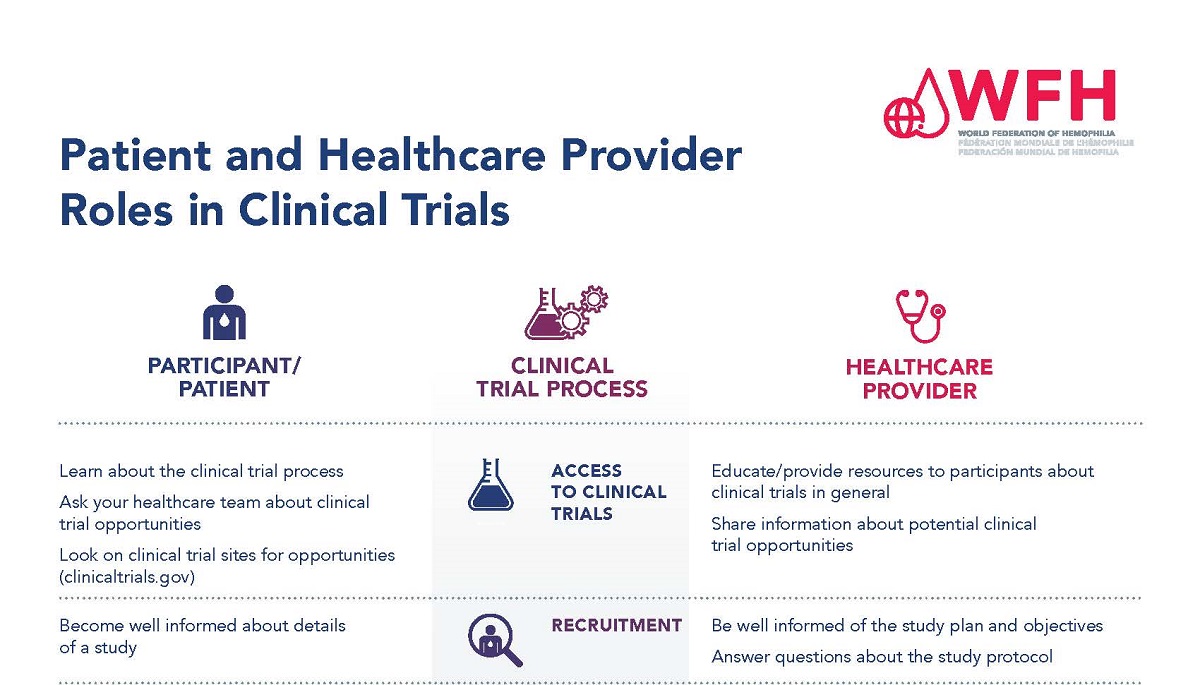
Participants in clinical trials and health care providers have different roles throughout the clinical trial process. It is important that
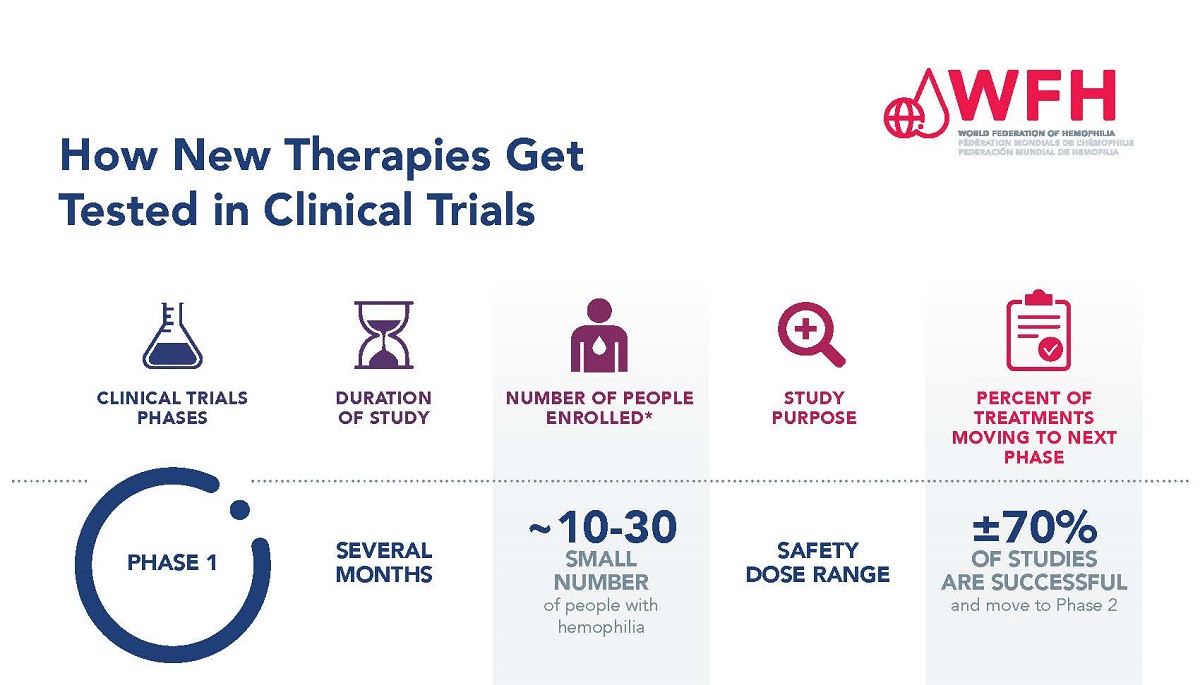
For a drug to become a treatment that doctors can prescribe, it must first be tested in a series of
Follow the progress of hemophilia gene therapy clinicals with the WFH Gene Therapy for Hemophilia Pipeline tool to track clinical

Clinical trials must be designed to fulfill high scientific and ethical standards. This monograph reviews the basic principles of good