Practical Education on Bleeding Disorders: Knowledge for All | WFH webinar series | April 22 2021

Listen to this 2-hour webinar presented on April 22, 2021 to learn about global topics related to bleeding disorders. The session was moderated by Glenn Pierce (WFH Vice President, Medical) and Marlène Beijlevelt (WFH Nurses Committee chair). Cedric Hermans (WFH Medical board member), Gianluigi Pasta (WFH MSK Committee chair), Sukesh Nair (Professor of laboratory haematology), […]
Non-factor Replacement Hemophilia Therapies

Adapted from: Global NMO Training 2018 plenary presentation by Glenn Pierce Reviewed by: Glenn Pierce Edited by: Georghia Michael The learner is strongly advised to complete Module 1 Introduction to Bleeding Disorders and Module 2 Evolution of Treatment Products, in the WFH Treatment Products eLearning Program before embarking upon Module 4. This fourth module in […]
Evolution of Treatment Products

Adapted from: Global NMO Training 2016 plenary presentation by Glenn Pierce Reviewed by: Glenn Pierce Edited by: Georghia Michael This second module in the Treatment Products eLearning Program explains the evolution of products for the treatment of bleeding disorders, from products derived from blood to artificially produced clotting factor concentrates (CFCs) with extended half-lives (EHL). […]
New Approaches to Tolerance: Oral Delivery of Blood Clotting Factors Bioencapsulated in Plant Cells

In the Monday morning session entitled Inhibitors: Clinical Aspects, Henry Daniell presents current advances in the development of new, affordable systems for oral delivery of clotting factors.
Guide for the Assessment of Clotting Factor Concentrates

The purpose of this guide is to inform and facilitate the selection and purchase of therapeutic products for the treatment of hemophilia. It discusses the factors that contribute to the quality, safety, and efficacy of hemophilia treatment products and, in particular, the provisions made for ensuring that these products are free of viruses. It covers […]
Proceedings of the WFH’s Tenth Global Forum on Research and Treatment Products for Bleeding Disorders

A summary of discussions held at the Tenth WFH Global Forum on Research and Treatment Products for Bleeding Disorders which brings together patient groups, healthcare providers, researchers, regulators, industry representatives, and not-for-profit fractionators.
Factor Replacement Therapy Schematic
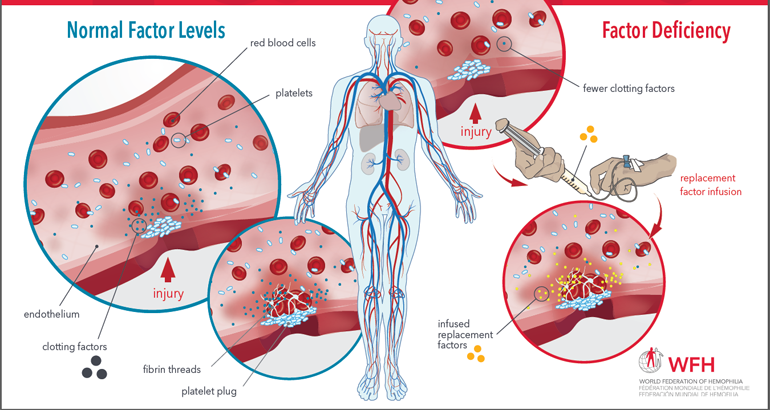
This illustrated schematic demonstrates the normal process of blood clotting, how it is diminished when a clotting factor is lacking, and how infusion of replacement therapy works to achieve normal levels of blood clotting. It also illustrates the clotting factor cascade and highlights the points in the cascade where each type of hemophilia treatment product […]
Essential Medicines List, Orphan Drugs, and Paid Plasma

In the Tuesday morning WFH session on global surveillance and collaboration, Brian O’Mahony, provides his vision for the role of the WFH in ensuring adequate access to a safe supply of hemophilia treatment.
Blood Safety and Donor Deferrals: The history and ongoing risk evaluation

In the Tuesday morning WFH session on global surveillance and collaboration, David Page, discusses donor deferrals, issues of blood safety and the role of the WFH.
Medical Plenary: Hemophilia treatment in 2030

In the Tuesday morning medical plenary, Erik Berntorp describes his vision of hemophilia treatment and medical advances in 2030.
WFH Webinar: Treatment Options for Hemophilia in the Developing World

On December 16, 2016, the WFH in collaboration with the ISTH, recorded a live interactive webinar on the challenges and opportunities of treatment options for hemophilia in the developing world, now available for viewing here. Introduced by Marijke van den Berg, Rolf Ljung opened with a summary of the lessons learned from decades of experience […]
Preventing Bleeds by Treatment: New era for hemophilia
In the Vice President Medical’s plenary of the WFH 2016 World Congress, Marijke van den Berg, discusses how bleeds may be prevented by treatment in a new era for hemophilia which she qualifies as exciting times.
